Resources for Professionals
Cure LBSL has put together comprehensive resources for clinicians, researchers, and educators who work with people with LBSL. Click any link below to find out more.
About LBSL
Leukoencephalopathy with Brainstem and Spinal Cord Involvement and Lactate Elevation (LBSL) is an ultra-rare, progressive neurological disorder that affects the brain and spinal cord. LBSL is caused by mutations in the DARS2 gene, which provides the body with instructions for making an enzyme called mitochondrial aspartyl-tRNA synthetase. As a result of mutations in DARS2, certain parts of nervous system do not have sufficient energy to function properly, affecting their function and the production of myelin.
Key Considerations When Working with Patients with LBSL
HEAD INJURY – Patients with LBSL are particularly vulnerable to severe consequences from head injury. Recommend thorough neurological assessment, extended observation, and low threshold for imaging.
AGGRAVATING CONDITIONS – Prevent whenever feasible, otherwise treat quickly and aggressively:
Fever/Infection
Dehydration
Fasting
Overheating
Hypothermia
INFECTION – Diligently look for source of fever or symptoms suggestive of infection; treat aggressively.
MEDICATION INTERACTIONS – Patients may be taking custom prescription “mito cocktails” (high potency antioxidants and amino acids) to support metabolic needs. Consult with pharmacist and/or clinicians familiar with mitochondrial disorders and treatment. Additional labwork may be indicated.
ASSESSMENTS – Patient vitals (especially body temperature), lab results, etc. may be out of reference range. Inquire about baseline, and trust patients/parents as experts on their own “normal” values.
PROTRACTED RECOVERY – Patient recovery may be longer than expected. Plan for extended impact from surgery, anesthesia, illness, injury, aggravating conditions (see above), and/or change in medication.
REFERRALS AND FOLLOW UP – Patients should be counseled to follow up with their primary care provider, neurologist, and/or metabolic specialist soon after discharge. Follow-up lab work may be indicated. Consult OT/PT as needed. Refer patient to new specialists as needed to complete care team.
Clinical Resources
Our LBSL Professional Resources document has up-to-date references for clinical and research professionals, including:
Genetic and Classification
Comprehensive Reviews
Referrals and Care Team
Conference Recaps
Patient Advocacy and Resources
Treatment Options
Supportive Care
Rehabilitative Medicine
OT / PT
Speech Therapy
Antiepileptics (as indicated for seizures)
Mitochondrial “Mito” Cocktail
To learn more about mitochondrial cocktails, please see the following professional presentations:
Review Article
Mitochondrial medicine therapies: rationale, evidence, and dosing guidelines
Authors: Barcelos I, Shadiack E, Ganetzky RD, Falk MJ.
Journal: Curr Opin Pediatr. 2020 Dec;32(6):707-718. 2022 LBSL Conference Recording
Speaker Dr. Hilary Vernon
Topic: Mitochondrial Cocktails: How to Access
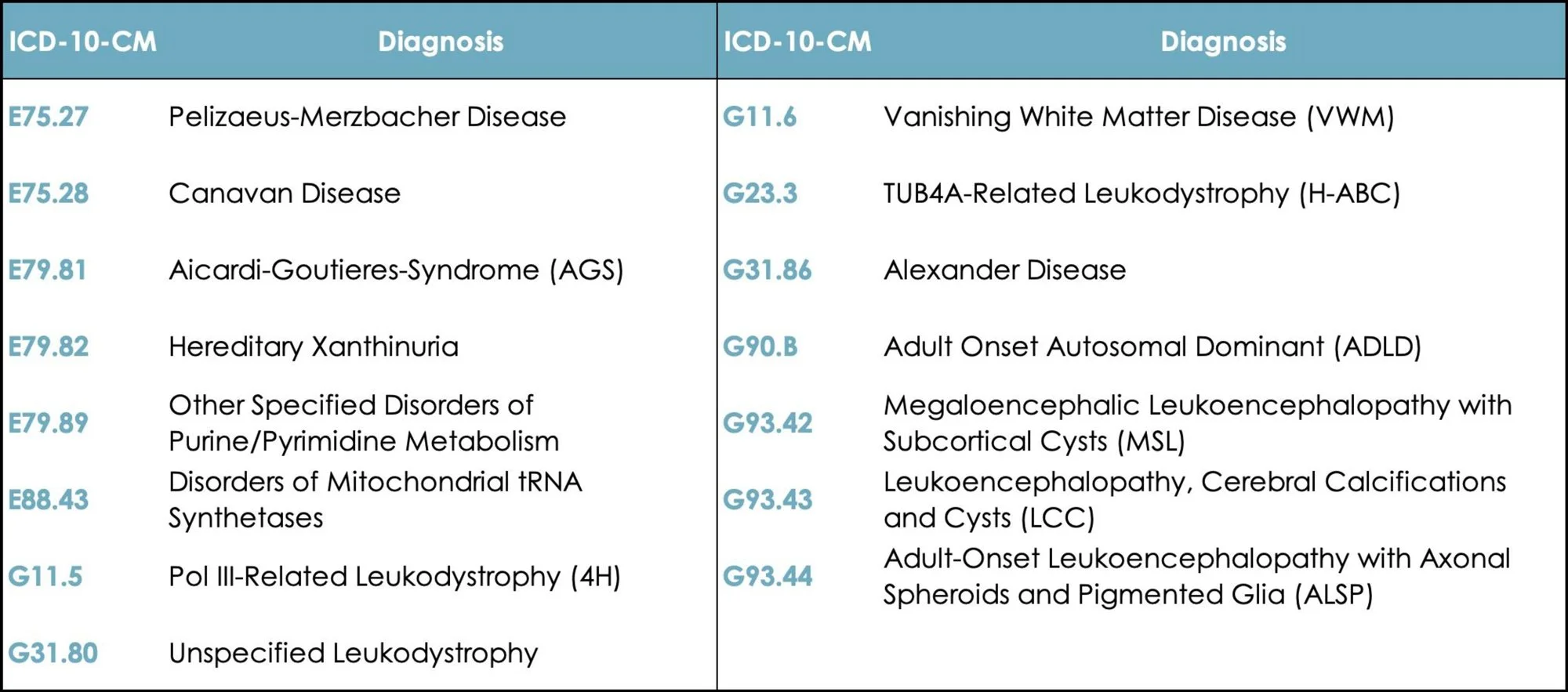
New ICD-10 Code for LBSL and Other Leukodystrophies
With enormous thanks to Josh Bonkowsky, MD, PhD and Monika Baker, MD (University of Utah), we are thrilled to announce the expansion of leukodystrophy ICD-10-CM codes, including for LBSL.
Effective October 1, 2023, LBSL is coded as: E88.43 Disorders of Mitochondrial tRNA Synthetases
Credit: University of Utah - https://uofuhealth.utah.edu/newsroom/news/2023/09/twelve-numbers-will-change-lives
Medical Protocols
These protocols were developed by Melody Kisor, MS and Beth McGinn from Cure LBSL, in close consultation with: Dr. Amena Smith Fine, MD PhD and Dr. S. Ali Fatemi, MD MBA, neurologists at Kennedy Krieger Institute; Dr. Kayla Kendric, MD from UCSF; and Dr. Mark Korson, MD from VMP Genetics. These guidelines are intended to educate and inform, but should never be used to replace clinical judgement. Whenever possible, collaborate with patient’s specialty care team and/or your referral network to determine optimal treatment plan.
Patient Care Guides
LBSL Patient Care Guide (coming soon)
School Resources
Thriving at School with LBSL – For classroom teachers and staff
School Needs & Accommodations – For special education and/or disability accommodation teams to create IEPs, 504s, or other accessibility plans
Comprehensive Reviews
Collaboration Opportunities
Cure LBSL supports and collaborates with clinicians, researchers, and patient advocacy groups from all over the globe. We partner with:
Kennedy Krieger Institute (Johns Hopkins Medicine)
Children’s Hospital of Philadelphia (USA)
Children’s Health of Orange County (USA)
Broad Institute of Harvard and MIT (USA)
Amsterdam University Medical Center (Netherlands)
University of Helsinki (Finland)
Hospital Pequeno Príncipe (Brazil)
Global Leukodystrophy Initiative
The Global Leukodystrophy Initiative Clinical Trials Network is a consortium of scientists, industry stakeholders and patient advocacy leaders working together to promote advances in the diagnosis and treatment of leukodystrophies. Click HERE for the the recording of the LBSL / HBSL Working Group presentation at the 2022 GLIA Scientific Meeting in Philadelphia.